Follicle-stimulating hormone
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Follicle-stimulating hormone (FSH) is a hormone found in humans and other animals. It is synthesized and secreted by gonadotrophs of the anterior pituitary gland. FSH regulates the development, growth, pubertal maturation, and reproductive processes of the body. FSH and Luteinizing hormone (LH) act synergistically in reproduction.
Contents |
Structure
FSH is a glycoprotein. Each monomeric unit is a protein molecule with a sugar attached to it; two of these make the full, functional protein. Its structure is similar to those of LH, TSH, and hCG. The protein dimer contains 2 polypeptide units, labeled alpha and beta subunits. The alpha subunits of LH, FSH, TSH, and hCG are identical, and contain 92 amino acids. The beta subunits vary. FSH has a beta subunit of 118 amino acids (FSHB), which confers its specific biologic action and is responsible for interaction with the FSH-receptor. The sugar part of the hormone is composed of fucose, galactose, mannose, galactosamine, glucosamine, and sialic acid, the latter being critical for its biologic half-life. The half-life of FSH is 3-4 hours. Its molecular wt is 30000.
Genes
The gene for the alpha subunit is located on chromosome 6p21.1-23. It is expressed in different cell types. The gene for the FSH beta subunit is located on chromosome 11p13, and is expressed in gonadotropes of the pituitary cells, controlled by GnRH, inhibited by inhibin, and enhanced by activin.
Activity
FSH regulates the development, growth, pubertal maturation, and reproductive processes of the human body.
- In both males and females, FSH stimulates the maturation of germ cells.
- In males, FSH induces Sertoli cells to secrete inhibin and stimulates the formation of sertoli-sertoli tight junctions (zonula occludens).
- In females, FSH initiates follicular growth, specifically affecting granulosa cells. With the concomitant rise in inhibin B, FSH levels then decline in the late follicular phase. This seems to be critical in selecting only the most advanced follicle to proceed to ovulation. At the end of the luteal phase, there is a slight rise in FSH that seems to be of importance to start the next ovulatory cycle.
Like its partner LH, FSH release at the pituitary gland is controlled by pulses of gonadotropin-releasing hormone (GnRH). Those pulses, in turn, are subject to the oestrogen feed-back from the gonads.
Effects in females
FSH stimulates the growth and recruitment of immature Ovarian follicles in the ovary. In early (small) antral follicles, FSH is the major survival factor that rescues the follicles from apoptosis (programmed death of the somatic cells of the follicle and oocyte). In the luteal-follicle phase transition period the serum levels of progesterone and estrogen (primarily estradiol) decrease and no longer suppress the release of FSH, consequently FSH peaks at about day three (day one is the first day of menstrual flow). The cohort of small antral follicles is normally sufficiently in number to produce enough Inhibin B to lower FSH serum levels.
In addition, there is evidence that gonadotrophin surge-attenuating factor produced by small follicles during the first half of the follicle phase also exerts a negative feedback on pulsatile luteinizing hormone (LH) secretion amplitude, thus allowing a more favorable environment for follicle growth and preventing premature luteinization.[1]
(As a woman nears perimenopause the number of small antral follicles recruited in each cycle diminishes and consequently insufficient Inhibin B is produced to fully lower FSH and the serum level of FSH begins to rise.)
When the follicle matures and reaches 8-10 mm in diameter it starts to secrete significant amounts of estradiol. Normally in humans only one follicle becomes dominant and survives to grow to 18-30 mm in size and ovulate, the remaining follicles in the cohort undergo atresia. The sharp increase in estradiol production by the dominant follicle (possibly along with a decrease in gonadotrophin surge-attenuating factor) cause a positive effect on the hypothalamus and pituitary and rapid GnRH pulses occur and an LH surge results.
The increase in serum estradiol levels cause a decrease in FSH production by inhibiting GnRH production in the hypothalamus.[2] The decrease in serum FSH level causes the smaller follicles in the current cohort to undergo atresia as they lack sufficient sensitivity to FSH to survive. Occasionally two follicles reach the 10 mm stage at the same time by chance and as both are equally sensitive to FSH both survive and grow in the low FSH environment and thus two ovulations can occur in one cycle possibly leading to non identical (dizygotic) twins.
Effects in males
FSH stimulates maturation of seminiferous tubules and spermatogenesis.
FSH enhances the production of androgen-binding protein by the Sertoli cells of the testes by binding to FSH receptors on their basolateral membranes,[3] and is critical for the initiation of spermatogenesis.
Measurement
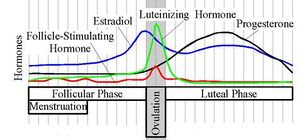
Follicle stimulating hormone is typically measured on day three of a woman's cycle when the levels of estradiol (E2) and progesterone are at the lowest point of the menstrual cycle.
Disease states
FSH levels are normally low during childhood and, in females, high after menopause.
High FSH levels

The most common reason for high serum FSH concentration is in a female who is undergoing or has recently undergone menopause. High levels of Follicle-Stimulating Hormone indicate that the normal restricting feedback from the gonad is absent, leading to an unrestricted pituitary FSH production.
If high FSH levels occur during the reproductive years, it is abnormal.
- Premature menopause also known as Premature Ovarian Failure
- Poor ovarian reserve also known as Premature Ovarian Aging
- Gonadal dysgenesis, Turner syndrome
- Castration
- Swyer syndrome
- Certain forms of CAH
- Testicular failure.
Most of these conditions are associated with subfertility and/or infertility. Therefore high FSH levels are an indication of subfertility and/or infertility.
Low FSH levels
Diminished secretion of FSH can result in failure of gonadal function (hypogonadism). This condition is typically manifested in males as failure in production of normal numbers of sperm. In females, cessation of reproductive cycles is commonly observed. Conditions with very low FSH secretions are:
- Polycystic Ovarian Syndrome
- Polycystic Ovarian Syndrome + Obesity + Hirsutism + Infertility
- Kallmann syndrome
- Hypothalamic suppression
- Hypopituitarism
- Hyperprolactinemia
- Gonadotropin deficiency
- Gonadal suppression therapy
- GnRH antagonist
- GnRH agonist (downregulation).
Availability
FSH is available mixed with LH activity in various menotropins including more purified forms of urinary gonadotropins such as Menopur, as well as without LH activity as recombinant FSH (Gonal F, Follistim, Follitropin alpha). It is used commonly in infertility therapy to stimulate follicular development, notably in IVF therapy, as well as with interuterine insemination (IUI). (See Gonadotropin Preparations.)
References
- ↑ Fowler PA, Sorsa-Leslie T , Harris W, Mason HD, 2003. “Ovarian gonadotrophin surge-attenuating factor (GnSAF): where are we after 20 years of research?” Reproduction, 126, 689–699.
- ↑ DiPiro, et al. Pharmacotherapy: A Pathophysiologic Approach, 2007. Chapter 82, page 1313.
- ↑ Page 1125 in: Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. pp. 1300. ISBN 1-4160-2328-3.
External links
- Day 3 FSH levels From the Infertility Blog by Dr. Fred Licciardi
- High FSH: an excuse to send patients away From the Infertility Blog by Dr. Fred Licciardi
- FSH and Estradiol
- Causes & Symptoms of High FSH
- Information on high FSH compiled by a woman with high FSH
|
|||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||